Shenzhen JYMed Technology Co., Ltd recently received an invention patent certificate issued by the European Patent Office for the “Synthesis Method of Liraglutide with Low Racemic Impurities.” This patent represents a new process for synthesizing liraglutide, which not only ensures stable yield but also significantly reduces the formation of the racemic impurity [D-Thr^5]-Liraglutide, which closely resembles the target product. This innovation enhances the overall product quality.
 The acquisition of this European patent demonstrates the comprehensive R&D capabilities of the company, further solidifying its technological advantages. It contributes to strengthening JYMed’s core competitiveness and expanding its presence in international markets. Additionally, it reinforces the company’s intellectual property advantages, helping to maintain and enhance its global market competitiveness.
The acquisition of this European patent demonstrates the comprehensive R&D capabilities of the company, further solidifying its technological advantages. It contributes to strengthening JYMed’s core competitiveness and expanding its presence in international markets. Additionally, it reinforces the company’s intellectual property advantages, helping to maintain and enhance its global market competitiveness.
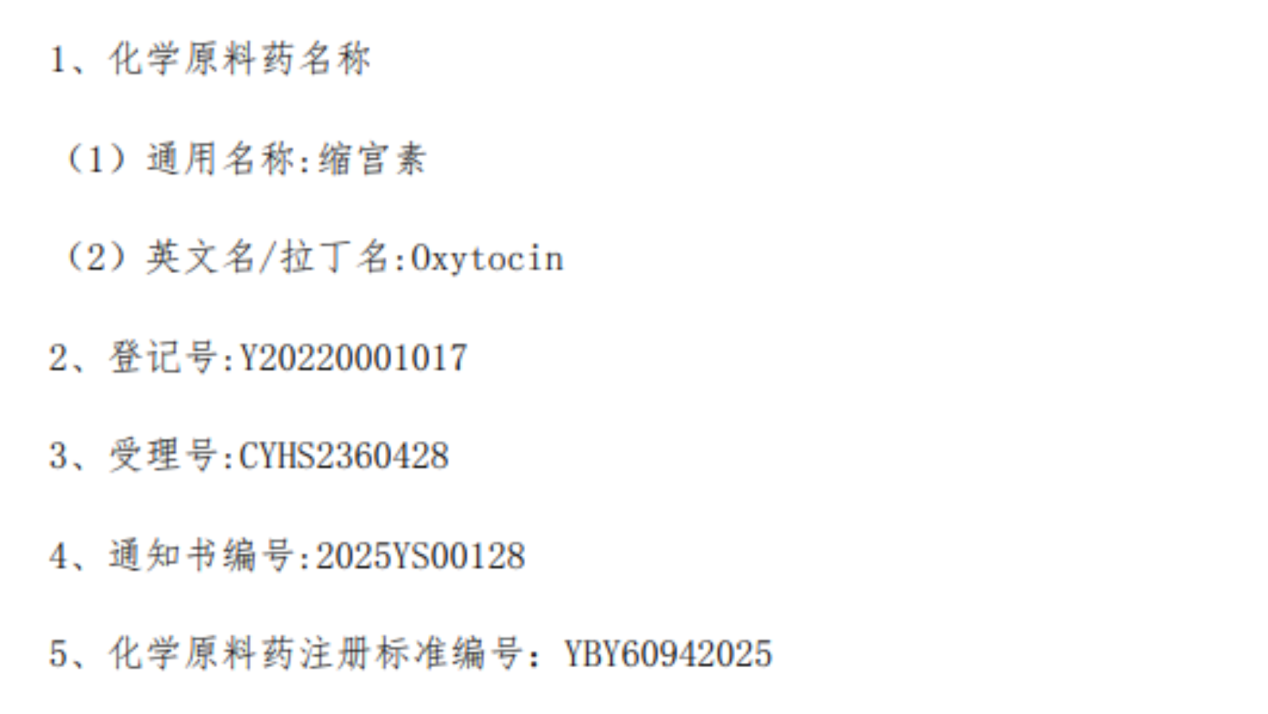
Hubei JXBio Pharmaceutical Co., Ltd, a subsidiary of Shenzhen JYMed Technology Co., Ltd, has recently received the Market Approval Notification for Oxytocin Active Pharmaceutical Ingredient (API) issued by the National Medical Products Administration (NMPA) of China.
This approval signifies that JXBio’s oxytocin API meets the regulatory technical requirements set by the national drug evaluation system. It marks an important milestone for the company, further enriching its product portfolio and providing a strong foundation for market expansion in the oxytocin sector.
ABOUT JYMED
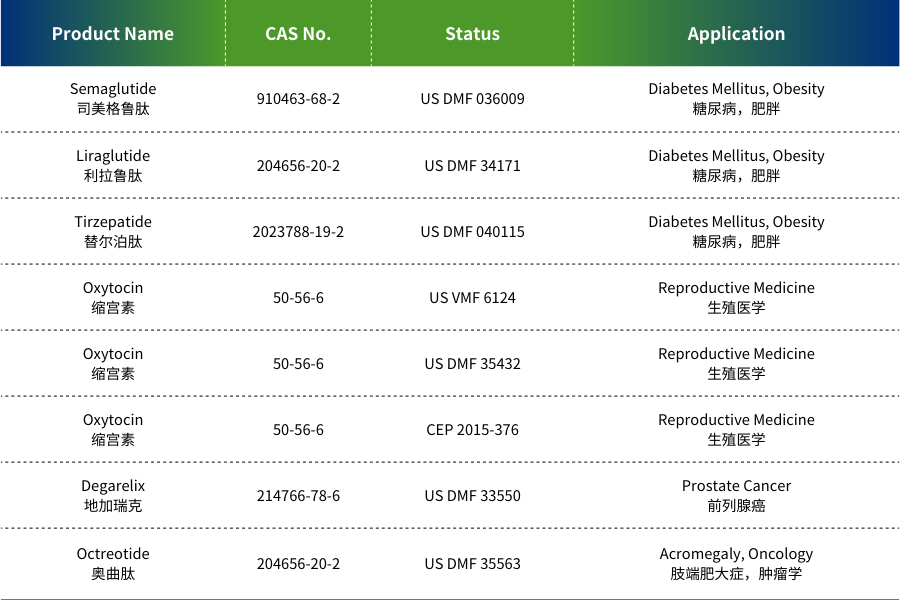
JYMed is a high-tech biopharmaceutical company specializing in the independent research, development, production, and sales of peptide-based products, as well as contract development and manufacturing organization (CDMO) services. The company is committed to providing high-quality peptide APIs and customized solutions to global clients. Its product portfolio includes dozens of peptide APIs, with core products such as Semaglutide and Terlipressin having already completed U.S. FDA DMF filings.
Its subsidiary,Hubei JXBio Pharmaceutical Co.,Ltd., operates state-of-the-art peptide API production lines that comply with cGMP standards set by the U.S. FDA, European EMA, and China’s NMPA. The facility includes 10 large-scale and pilot production lines and has established a rigorous pharmaceutical quality management system (QMS) and an environmental health and safety (EHS) management system. These ensure that the entire process, from R&D to production, meets the highest international standards. The company has successfully passed GMP compliance inspections by both the U.S. FDA and China’s NMPA and has been recognized by leading global pharmaceutical companies for its EHS management excellence, demonstrating its outstanding commitment to quality, safety, and environmental responsibility.
Core Business Areas:Domestic and international peptide API registration and compliance,Veterinary and cosmetic peptides,Custom peptide services, including CRO, CMO, and OEM solutions,Peptide-drug conjugates (PDCs), including peptide-radionuclide, peptide-small molecule, peptide-protein, and peptide-RNA therapeutics.
MAIN PRODUCTS
For more details on our products, please contact us.
Global API and Cosmetic Inquiries: Tel No.: +86-15013529272;
API Registration & CDMO Services (USA EU market): +86-15818682250
E-mail: jymed@jymedtech.com
Address: Floors 8 & 9, Building 1, Shenzhen Biomedical Innovation Industrial Park, 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Post time: Mar-31-2025