
On October 12, 2024, JYMed’s Liraglutide API obtained the Written Confirmation (WC) certificate, marking a critical step toward the successful export of the API to the EU market.

The WC (Written Confirmation) is a mandatory requirement for the export of APIs from non-EU countries to the EU market. Issued by the regulatory authority of the exporting country, this certificate ensures that the exported API complies with the Good Manufacturing Practice (GMP) standards set by the EU. It plays a vital role in ensuring the quality and safety of APIs and is essential for non-EU countries seeking access to the EU pharmaceutical market.


The receipt of WC certification for Liraglutide API not only reflects official recognition of the quality and safety of JYMed’s products but also enhances the company’s capability to expand its presence in the EU API market. This achievement strengthens JYMed’s position in the global pharmaceutical industry, providing greater development opportunities and increasing its international reputation.
About JYMed

Shenzhen JYMed Technology Co., Ltd. (hereinafter referred to as JYMed) was established in 2009, specializing in the research, development, production, and sales of peptides and peptide-related products. With one research center and three major production bases, JYMed is one of the largest producers of chemically synthesized peptide APIs in China. The company's core R&D team boasts over 20 years of experience in the peptide industry and has successfully passed FDA inspections twice. JYMed’s comprehensive and efficient peptide industrialization system offers customers a full range of services, including the development and production of therapeutic peptides, veterinary peptides, antimicrobial peptides, and cosmetic peptides, as well as registration and regulatory support.
Main Business Activities
1.Domestic and international registration of peptide APIs
2.Veterinary and cosmetic peptides
3.Custom peptides and CRO, CMO, OEM services
4.PDC drugs (peptide-radionuclide, peptide-small molecule, peptide-protein, peptide-RNA)
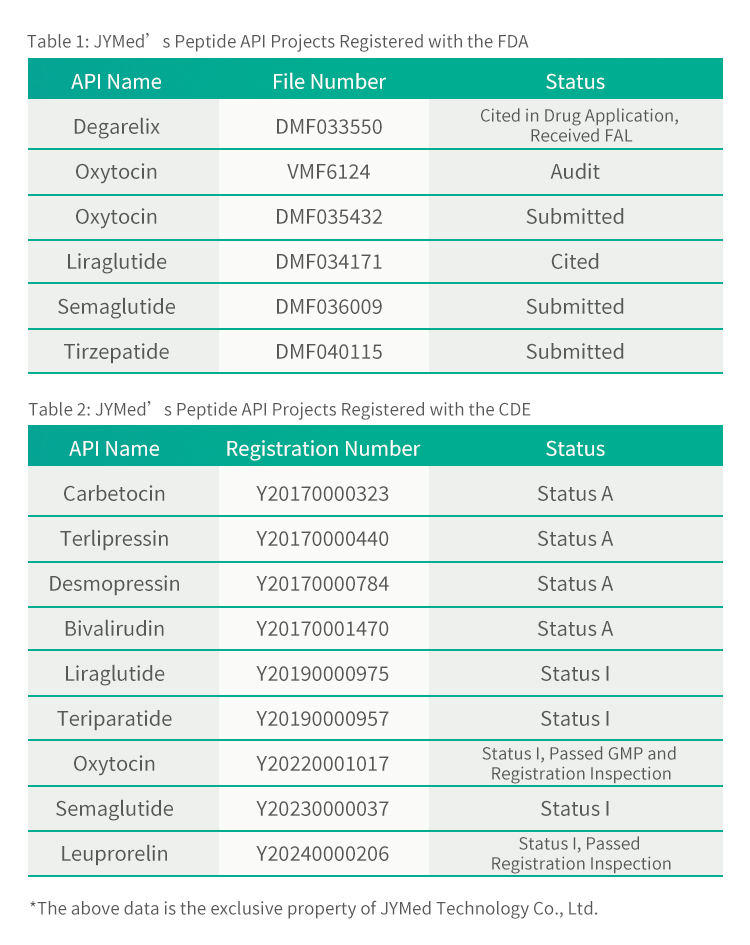
In addition to Tirzepatide, JYMed has submitted registration filings with the FDA and CDE for several other API products, including the currently popular GLP-1RA class drugs such as Semaglutide and Liraglutide. Future customers using JYMed's products will be able to directly reference the CDE registration number or DMF file number when submitting registration applications to the FDA or CDE. This will significantly reduce the time required for preparing application documents, as well as the evaluation time and cost of product review.
Contact Us


Shenzhen JYMed Technology Co., Ltd.
Address: 8th & 9th Floors, Building 1, Shenzhen Biomedical Innovation Industrial Park, No. 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Phone: +86 755-26612112
Website: http://www.jymedtech.com/
Post time: Oct-17-2024







