
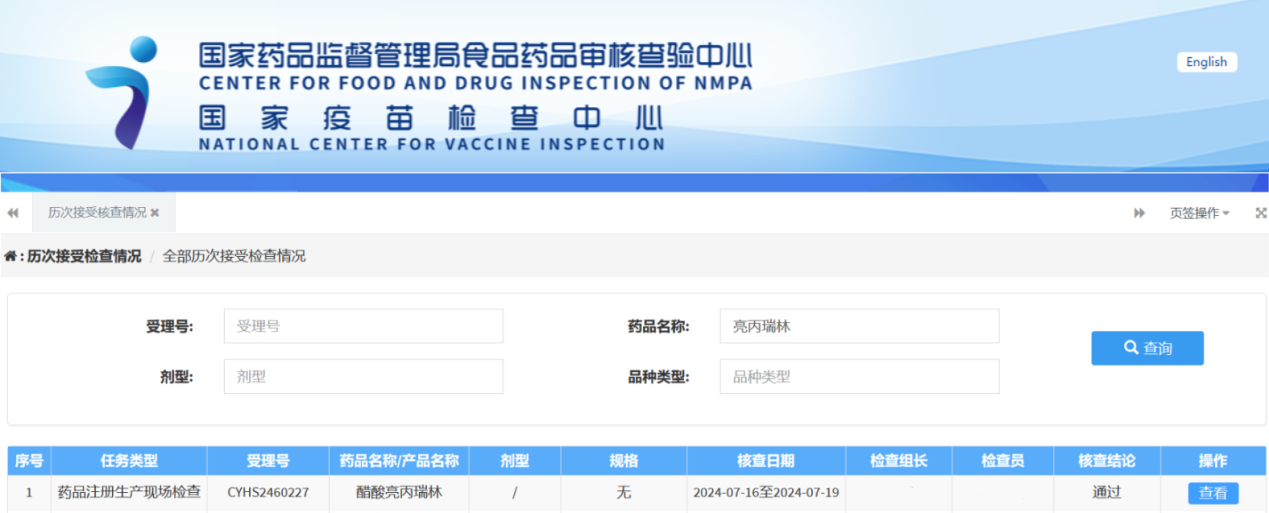
Recently, JYMed Technology Co., Ltd. announced that Leuprorelin Acetate, produced by its subsidiary Hubei JX Bio-Pharmaceutical Co., Ltd., has successfully passed the drug registration inspection.
Original Drug Market Overview
Leuprorelin Acetate is an injectable medication used to treat hormone-dependent diseases, with the molecular formula C59H84N16O12•xC2H4O2. It is a gonadotropin-releasing hormone agonist (GnRHa) that works by inhibiting the pituitary-gonadal system. Originally co-developed by AbbVie and Takeda Pharmaceutical, this drug is marketed under different brand names in various countries. In the United States, it is sold under the brand name LUPRON DEPOT, while in China, it is marketed as Yina Tong.
Clear Process and Well-Defined Roles
From 2019 to 2022, the pharmaceutical research and development were completed, followed by the registration of the API in March 2024, when the acceptance notice was received. The drug registration inspection was passed in August 2024. JYMed Technology Co., Ltd. was responsible for the process development, analytical method development, impurity studies, structure confirmation, and method validation. Hubei JX Bio-Pharmaceutical Co., Ltd. was in charge of the process validation production, analytical method validation, and stability studies for the API.
Expanding Market and Growing Demand
The rising incidence of prostate cancer and uterine fibroids is driving the increased demand for Leuprorelin Acetate. The North American market currently dominates the Leuprorelin Acetate market, with growing healthcare expenditures and high acceptance of new technologies being the primary growth drivers. Simultaneously, the Asian market, particularly China, is also showing strong demand for Leuprorelin Acetate. Due to its effectiveness, global demand for this drug is on the rise, with the market size expected to reach USD 3,946.1 million by 2031, reflecting a compound annual growth rate (CAGR) of 4.86% from 2021 to 2031.
About JYMed

Shenzhen JYMed Technology Co., Ltd. (hereinafter referred to as JYMed) was established in 2009, specializing in the research, development, production, and sales of peptides and peptide-related products. With one research center and three major production bases, JYMed is one of the largest producers of chemically synthesized peptide APIs in China. The company's core R&D team boasts over 20 years of experience in the peptide industry and has successfully passed FDA inspections twice. JYMed’s comprehensive and efficient peptide industrialization system offers customers a full range of services, including the development and production of therapeutic peptides, veterinary peptides, antimicrobial peptides, and cosmetic peptides, as well as registration and regulatory support.
Main Business Activities
1.Domestic and international registration of peptide APIs
2.Veterinary and cosmetic peptides
3.Custom peptides and CRO, CMO, OEM services
4.PDC drugs (peptide-radionuclide, peptide-small molecule, peptide-protein, peptide-RNA)
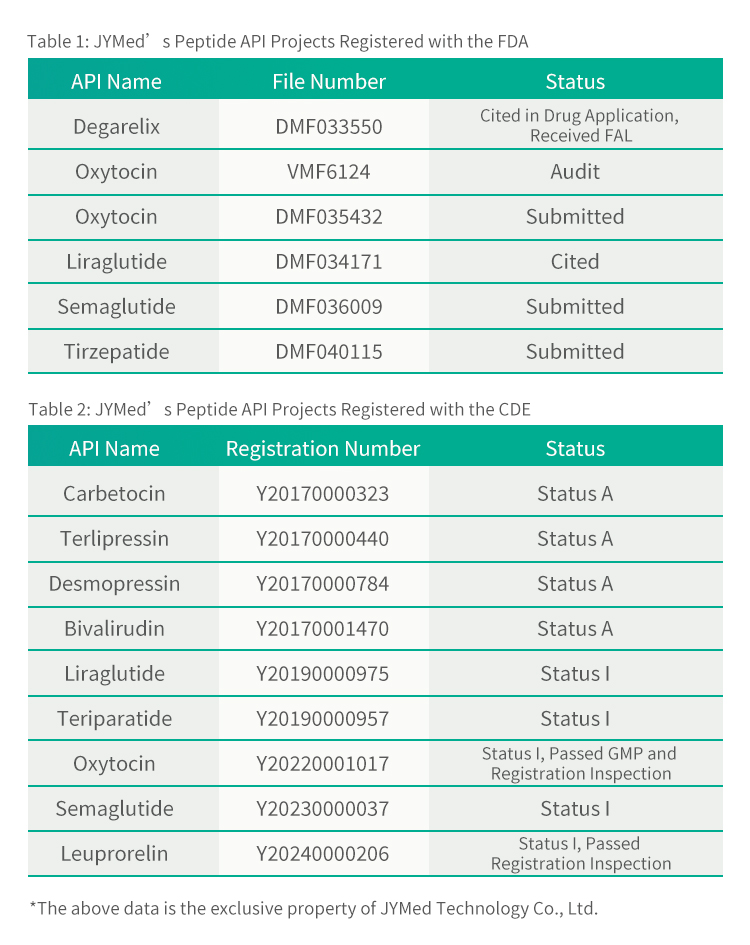
In addition to Leuprorelin Acetate, JYMed has submitted registration filings with the FDA and CDE for several other API products, including the currently popular GLP-1RA class drugs such as Semaglutide、 Liraglutide and Tirzepatide. Future customers using JYMed's products will be able to directly reference the CDE registration number or DMF file number when submitting registration applications to the FDA or CDE. This will significantly reduce the time required for preparing application documents, as well as the evaluation time and cost of product review.
Contact Us


Shenzhen JYMed Technology Co., Ltd.
Address: 8th&9th Floors, Building 1, Shenzhen Biomedical Innovation Industrial Park, No. 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Phone: +86 755-26612112
Website: http://www.jymedtech.com/
Post time: Aug-29-2024







