

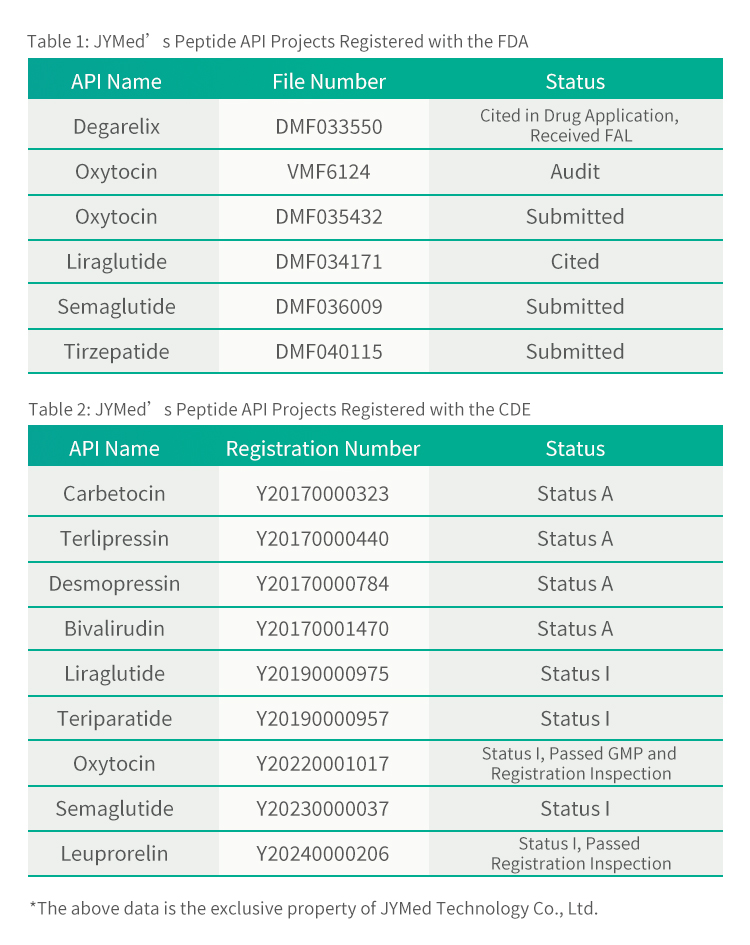
JYMed Technology Co., Ltd. is pleased to announce that its product, Tirzepatide, has successfully completed the Drug Master File (DMF) registration with the U.S. FDA (DMF Number: 040115) and received the FDA's Acknowledgement on August 2, 2024.
Mass Production with Stable Quality
According to JYMed Technology’s senior management, the bulk production of Tirzepatide Active Pharmaceutical Ingredient (API) can reach kilogram levels. The production batches are stable and continuous, with minimal variation between batches, ensuring consistent quality.
Significant Effects on Glucose and Lipid Reduction
Tirzepatide is the world’s first approved once-weekly GIP/GLP-1 receptor agonist. As a dual receptor agonist, it can simultaneously bind and activate both the glucose-dependent insulinotropic polypeptide (GIP) receptor and the GLP-1 receptor in the human body. In addition to lowering glucose levels, it reduces food intake, body weight, and fat content, and regulates lipid utilization. Beyond its significant glucose-lowering and weight-reducing effects, subgroup analyses from the SURPASS series of studies have shown that Tirzepatide also improves metabolic indicators such as blood pressure, blood lipids, BMI, and waist circumference.
Multinational Approvals and Promising Prospects
According to relevant information, the glucose-lowering Mounjaro was first approved by the U.S. FDA in May 2022 for the treatment of adults with type 2 diabetes. It has subsequently received approvals in the EU, Japan, and other regions. In November 2023, the FDA also approved the weight loss indication under the brand name Zepbound. In May 2024, it successfully entered the Chinese market. Given its broad application prospects and strong supporting research data, Tirzepatide has become one of the most prominent peptide drugs today. Its sales reached $5.163 billion in 2023, and the first quarter of 2024 alone saw sales of $2.324 billion, demonstrating an astonishing growth rate.
About JYMed

Shenzhen JYMed Technology Co., Ltd. (hereinafter referred to as JYMed) was established in 2009, specializing in the research, development, production, and sales of peptides and peptide-related products. With one research center and three major production bases, JYMed is one of the largest producers of chemically synthesized peptide APIs in China. The company's core R&D team boasts over 20 years of experience in the peptide industry and has successfully passed FDA inspections twice. JYMed’s comprehensive and efficient peptide industrialization system offers customers a full range of services, including the development and production of therapeutic peptides, veterinary peptides, antimicrobial peptides, and cosmetic peptides, as well as registration and regulatory support.
Main Business Activities
1.Domestic and international registration of peptide APIs
2.Veterinary and cosmetic peptides
3.Custom peptides and CRO, CMO, OEM services
4.PDC drugs (peptide-radionuclide, peptide-small molecule, peptide-protein, peptide-RNA)
In addition to Tirzepatide, JYMed has submitted registration filings with the FDA and CDE for several other API products, including the currently popular GLP-1RA class drugs such as Semaglutide and Liraglutide. Future customers using JYMed's products will be able to directly reference the CDE registration number or DMF file number when submitting registration applications to the FDA or CDE. This will significantly reduce the time required for preparing application documents, as well as the evaluation time and cost of product review.
Contact Us


Shenzhen JYMed Technology Co., Ltd.
Address: 8th & 9th Floors, Building 1, Shenzhen Biomedical Innovation Industrial Park, No. 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Phone: +86 755-26612112
Website: http://www.jymedtech.com/
Post time: Aug-12-2024







