Nuper, officina productionis peptidorum JYMed, Hubei Jianxiang Biopharmaceutical Co., Ltd., duo documenta publica ab Administratione Medicamentorum Provinciali Hubei edita accepit: "Notificationem Resultatus Inspectionis Conformitatis GMP Medicamentorum" (No. E GMP 2024-258 et No. E GMP 2024-260) et "Certificatum Exportationis ad EU Ingredientium Pharmaceuticorum Activorum (API)" (Certificatum WC, No. HB240039).
Haec documenta confirmant lineam productionis A102 in Officina A102 (ad productionem API Oxytocini et Semaglutidi) et lineam productionis A092 in Officina A092 (ad productionem API Terlipressini) apud Hubei Jianxiang normas GMP Sinarum satisfacere, quae requisitis GMP Unionis Europaeae, Organizationis Mundialis Salutis (OMS), et ICH Q7 pro pharmaceuticis aequipollent.
Inspectio cum obsequio finita est, indicans Hubei Jianxiang administrationem qualitatis productionis et rationes regulatrices altis normis domesticis satisfacere. Haec progressio expansionem Hubei Jianxiang in foro globali, praesertim in foro Unionis Europaeae, adiuvabit, fiduciam clientium augebit, collaborationes internationales promovebit, et ad incrementum distributionemque globalem medicamentorum peptidi fundatorum conferet. Crescente postulatione fori internationalis, Hubei Jianxiang melius se collocabit ut necessitatibus clientium globalium cum productis et officiis altae qualitatis satisfaciat.
De JYMed
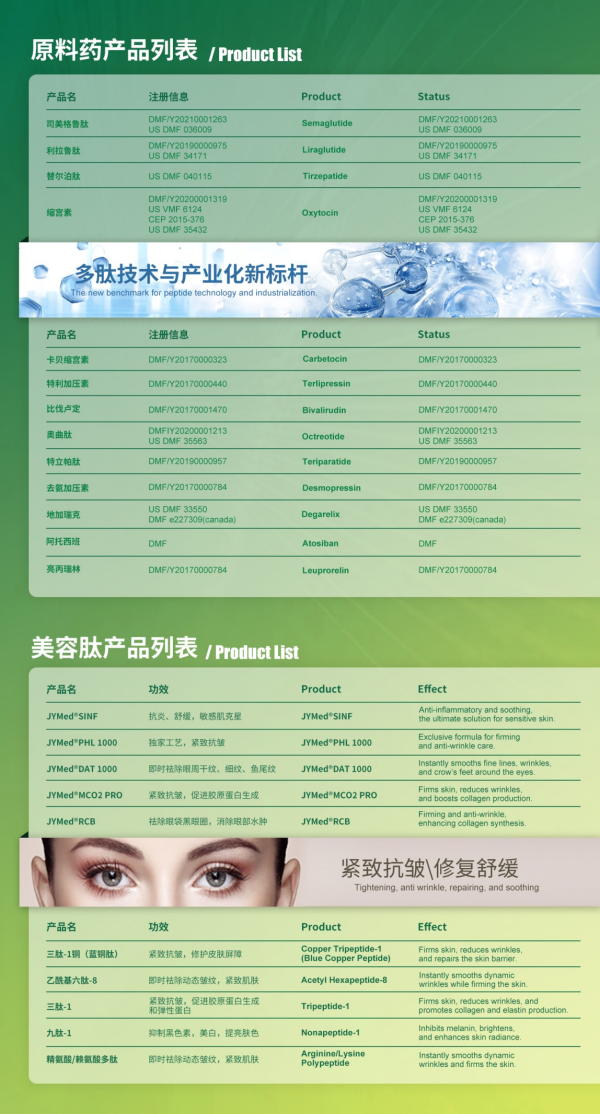
Anno MMIX condita, Shenzhen JYMed Technology Co., Ltd. est societas biotechnologica quae in investigatione, evolutione, productione, et venditione productorum peptidicorum independentibus specializatur, una cum officiis singularibus investigationis et evolutionis peptidicorum et fabricationis. Societas plus quam XX API peptidicorum offert, cum quinque productis, inter quas Semaglutide et Tirzepatide, feliciter licentias DMF apud FDA Civitatum Foederatarum perfecerit.
Officina Hubei JX decem lineas productionis pro peptidis API (inter quas lineae scalae pilotae) habet, quae cum normis cGMP Civitatum Foederatarum, Unionis Europaeae, et Sinarum congruunt. Officina systema comprehensivum administrationis qualitatis pharmaceuticae et systema administrationis EHS (Ambientalis, Salutis, et Securitatis) administrat. Inspectiones officiales GMP NMPA et revisiones EHS a clientibus praestantibus globalibus peractas superavit.
Officia Primaria
- Registratio API peptidorum domestica et internationalis
- Peptida veterinaria et cosmetica
- Synthesis peptidorum ad usum clientium, CRO, CMO, et officia OEM
- PDC (Coniuga Peptidorum Medicamentorum), inter quae coniuga peptido-radionuclido, peptido-molecula parva, peptido-proteino, et peptido-RNA.
Informationes Contactus
Inscriptio:Tabulata octava et nona, Aedificium 1, Shenzhen Biomedical Innovation Industrial Park, Jin Hui Road 14, Kengzi Street, Pingshan District, Shenzhen, Sina.
Pro interrogationibus API internationalibus:
+86-755-26612112 | +86-15013529272
Pro Materiis Crudis Peptidorum Cosmeticorum Domesticarum:
+86-755-26612112 | +86-15013529272
Pro Registratione API Domestica et Servitiis CDMO:
+86-15818682250
Situs interretialis: www.jymedtech.com
Tempus publicationis: Ian-X-MMXV