
Die XII mensis Octobris, anno MMXXIV, medicamentum Liraglutidi API societatis JYMed certificatum Confirmationis Scriptae (WC) obtinuit, quod gradum criticum ad exportationem prosperam medicamenti API in mercatum Unionis Europaeae significavit.

TheConfirmatio Scripta (WC)requisitum est necessarium ad exportationem API ex terris extra Unionem Europaeam in mercatum Unionis Europaeae. Ab auctoritate regulatoria terrae exportatricis emissa, haec certificatio efficit ut API exportata cum...Bonae Praxis Fabricationis (GMP)normas ab Unione Europaea statutas. Munus vitale agit in qualitate et salute API curanda et essentiale est nationibus extra Unionem Europaeam aditum ad mercatum pharmaceuticum Unionis Europaeae petentibus.


Acceptio certificationis WC pro Liraglutide API non solum agnitionem officialem qualitatis et salutis productorum JYMed reflectit, sed etiam facultatem societatis ad praesentiam suam in foro API Unionis Europaeae amplificandam auget. Hoc decus positionem JYMed in industria pharmaceutica globali firmat, maiores opportunitates progressionis praebens et famam eius internationalem augens.
De JYMed

Societas technologica Shenzhen JYMed (deinceps JYMed appellata) anno 2009 condita est, in investigatione, evolutione, productione, et venditione peptidorum et productorum peptido conexorum specializata. Cum uno centro investigationis et tribus maioribus basibus productionis, JYMed est unus ex maximis productoribus API peptidorum chemice synthesizatorum in Sinis. Turma principalis societatis in investigatione et evolutione plus quam viginti annos experientiae in industria peptidorum gloriatur et bis inspectiones FDA feliciter superavit. Systema industrializationis peptidorum comprehensivum et efficax JYMed clientibus seriem plenam servitiorum offert, inter quas evolutio et productio peptidorum therapeuticorum, peptidorum veterinariorum, peptidorum antimicrobialium, et peptidorum cosmeticorum, necnon subsidium registrationis et regulationis.
Actiones Negotiales Principales
1. Registratio domestica et internationalis API peptidicorum
2. Peptida veterinaria et cosmetica
3. Peptida ad usum accommodata et officia CRO, CMO, OEM
4. Medicamenta PDC (peptidum-radionuclidum, peptidum-molecula parva, peptidum-proteinum, peptidum-RNA)
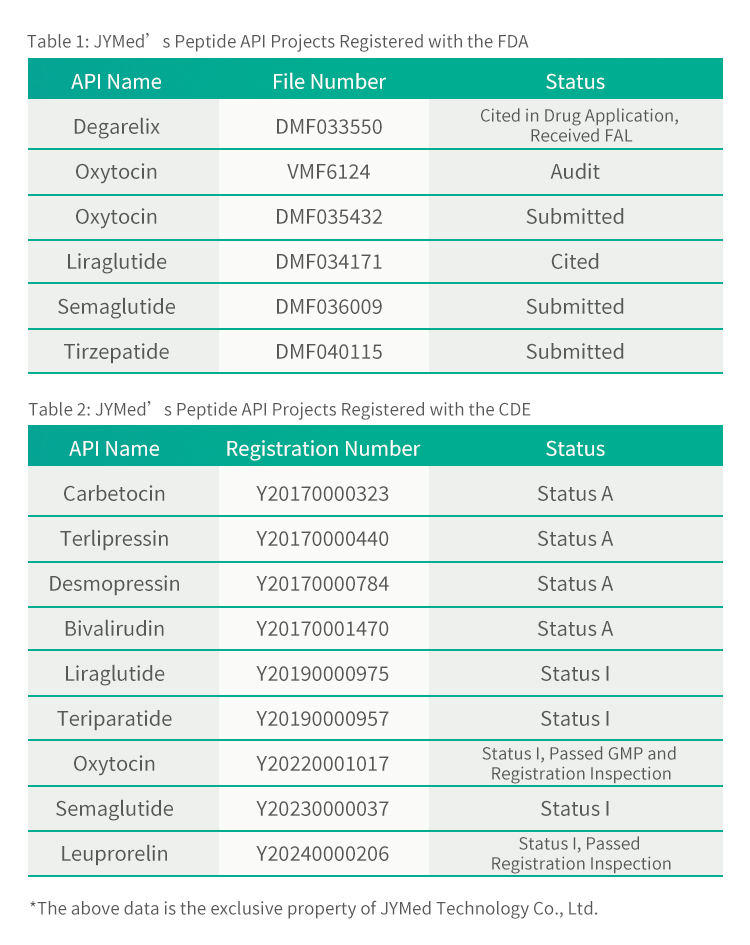
Praeter Tirzepatidum, JYMed inscriptiones apud FDA et CDE pro pluribus aliis productis API, inter quas medicamenta classis GLP-1RA nunc popularia, ut Semaglutidum et Liraglutidum, submisit. Futuri clientes qui producta JYMed utuntur, numerum inscriptionis CDE vel numerum fasciculi DMF directe consulere poterunt cum inscriptiones ad FDA vel CDE submittunt. Hoc tempus ad documenta inscriptionis paranda requisitum, necnon tempus aestimationis et sumptum recensionis producti, significanter minuet.
Contacta Nos


Shenzhen JYMed Technologiae Societas, Ltd.
Inscriptio: Tabulata Octava et Nona, Aedificium I, Shenzhen Biomedical Innovation Industrial Park, Via Jinhui No. 14, Subdistrictus Kengzi, Districtus Pingshan, Shenzhen.
Telephonum: +86 755-26612112
Situs interretialis:http://www.jymedtech.com/
Tempus publicationis: Oct-XVII-MMXXIV







