
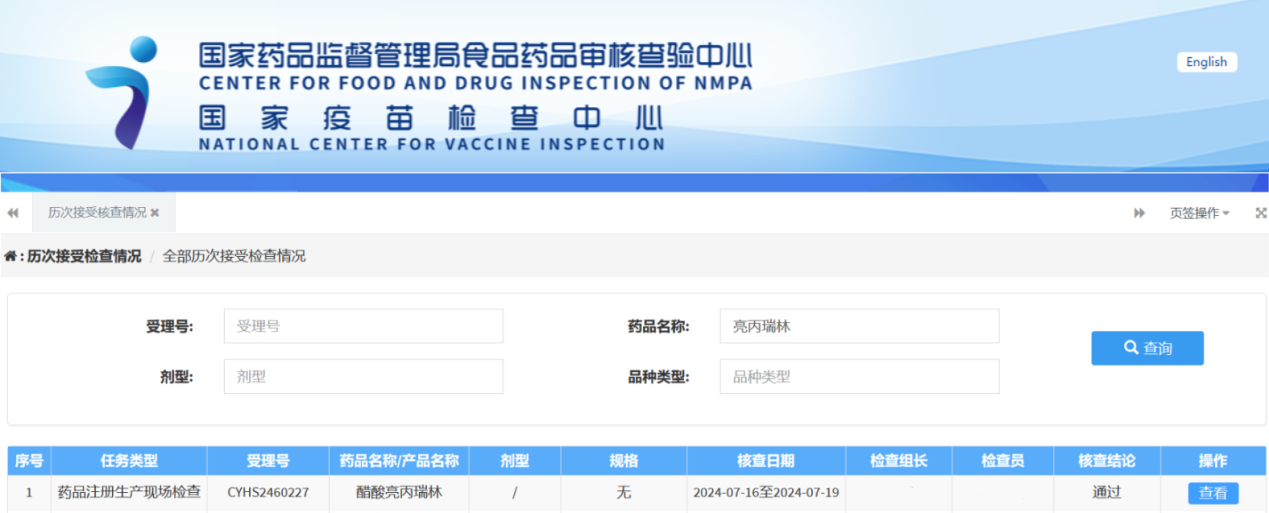
Nuper, JYMed Technology Co., Ltd. nuntiavit Leuprorelin Acetas, a subsidiaria sua Hubei JX Bio-Pharmaceutical Co., Ltd. productum, inspectionem registrationis medicamenti feliciter superasse.
Conspectus Mercatus Medicamentorum Originalis
Leuprorelini Acetas est medicamentum iniectabile ad morbos hormono-dependentes curandos adhibitum, formula moleculari C59H84N16O12•xC2H4O2. Est agonista hormonis gonadotropinum liberantis (GnRHa) qui systema pituitarium-gonadale inhibendo operatur. Initio ab AbbVie et Takeda Pharmaceutical coniunctim elaboratum, hoc medicamentum sub variis nominibus commercialibus in variis terris venditur. In Civitatibus Foederatis Americae, sub nomine commerciali LUPRON DEPOT venditur, dum in Sinis, ut Yina Tong venditur.
Processus Clarus et Munera Bene Definita
Ab anno MMXIX ad MMXXII, investigatio et progressus pharmaceutici completa sunt, deinde registratio substantiae activae (API) mense Martio MMXXIV facta est, cum notitia acceptationis accepta est. Inspectio registrationis medicamenti mense Augusto MMXXIV superata est. Societas JYMed Technology Co., Ltd. progressionem processus, progressionem methodi analyticae, studia impuritatum, confirmationem structurae, et validationem methodi curavit. Societas Hubei JX Bio-Pharmaceutical Co., Ltd. validationem processus, productionem, validationem methodi analyticae, et studia stabilitatis API curavit.
Mercatus Expandens et Crescens Postulatio
Crescens incidentia cancri prostatae et fibromatum uteri auget postulationem Leuprorelini Acetatis. Mercatus Americae Septentrionalis nunc dominatur mercatum Leuprorelini Acetatis, cum crescentibus impensis curationis et alta acceptatione novarum technologiarum praecipuis impulsoribus incrementi sint. Simul, mercatus Asiaticus, praesertim Sina, etiam validam postulationem Leuprorelini Acetatis ostendit. Propter eius efficaciam, postulatio globalis huius medicamenti crescit, cum magnitudo mercatus ad USD 3,946.1 miliones pervenire exspectatur anno 2031, quod reflectit compositum incrementum annuum (CAGR) 4.86% ab anno 2021 ad 2031.
De JYMed

Societas technologica Shenzhen JYMed (deinceps JYMed appellata) anno 2009 condita est, in investigatione, evolutione, productione, et venditione peptidorum et productorum peptido conexorum specializata. Cum uno centro investigationis et tribus maioribus basibus productionis, JYMed est unus ex maximis productoribus API peptidorum chemice synthesizatorum in Sinis. Turma principalis societatis in investigatione et evolutione plus quam viginti annos experientiae in industria peptidorum gloriatur et bis inspectiones FDA feliciter superavit. Systema industrializationis peptidorum comprehensivum et efficax JYMed clientibus seriem plenam servitiorum offert, inter quas evolutio et productio peptidorum therapeuticorum, peptidorum veterinariorum, peptidorum antimicrobialium, et peptidorum cosmeticorum, necnon subsidium registrationis et regulationis.
Actiones Negotiales Principales
1. Registratio domestica et internationalis API peptidicorum
2. Peptida veterinaria et cosmetica
3. Peptida ad usum accommodata et officia CRO, CMO, OEM
4. Medicamenta PDC (peptidum-radionuclidum, peptidum-molecula parva, peptidum-proteinum, peptidum-RNA)
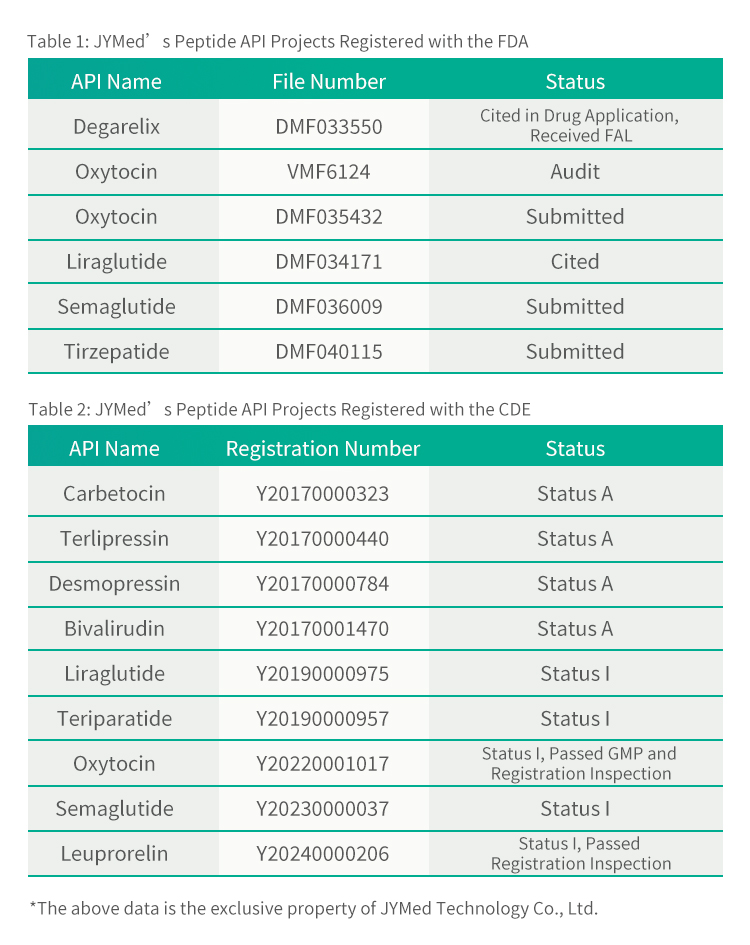
Praeter Leuprorelin Acetas, JYMed inscriptiones apud FDA et CDE pro pluribus aliis productis API, inter quas medicamenta classis GLP-1RA nunc popularia, ut Semaglutide, Liraglutide et Tirzepatide, submisit. Futuri clientes productis JYMed utentes numerum inscriptionis CDE vel numerum fasciculi DMF directe consulere poterunt cum inscriptiones ad FDA vel CDE submittunt. Hoc tempus ad documenta applicationis paranda requisitum, necnon tempus aestimationis et sumptum recensionis producti significanter minuet.
Contacta Nos


Shenzhen JYMed Technologiae Societas, Ltd.
Inscriptio:Tabulata octava et nona, Aedificium 1, Shenzhen Biomedical Innovation Industrial Park, Via Jinhui 14, Subdistrictus Kengzi, Districtus Pingshan, Shenzhen.
Telephonum:+86 755-26612112
Situs interretialis:http://www.jymedtech.com/
Tempus publicationis: XXIX Augusti, MMXXIV







