

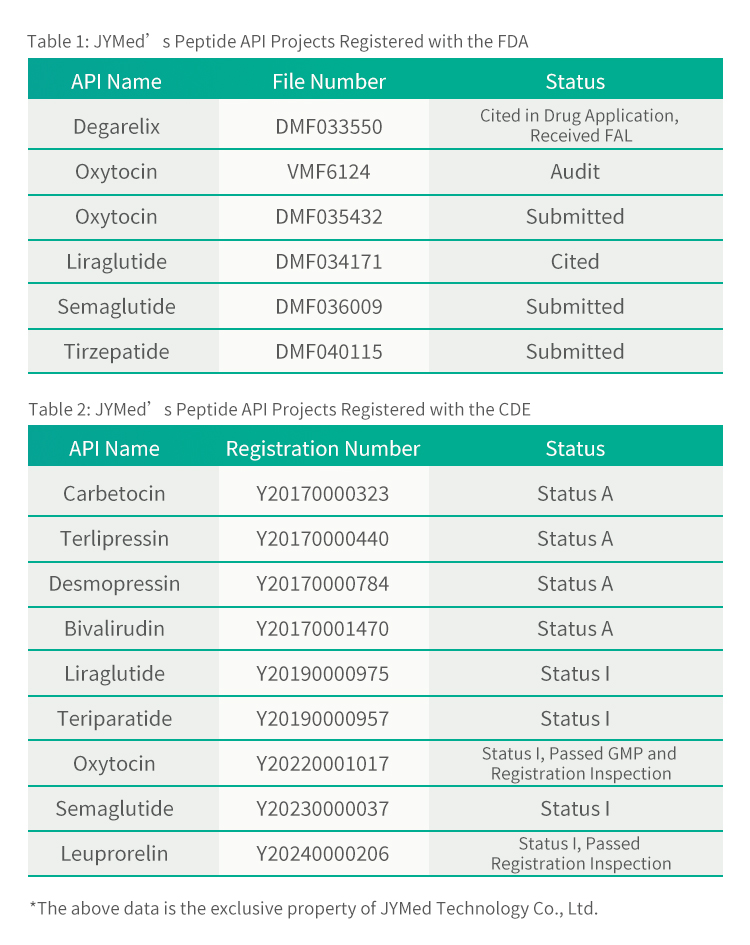
Societas JYMed Technology Co., Ltd. laeta nuntiat productum suum, Tirzepatidum, feliciter registrationem Fasciculi Magistri Medicamentorum (DMF) apud FDA Civitatum Foederatarum (Numerus DMF: 040115) perfecisse et recognitionem FDA die 2 Augusti 2024 accepisse.
Productio Massae cum Qualitate Stabili
Secundum administratores superiores JYMed Technology, productio magna Tirzepatidi Ingredientis Pharmaceutici Activi (API) ad gradus chiliogrammatum pervenire potest. Series productionis stabiles et continuae sunt, cum minima variatione inter series, qualitatem constantem praebentes.
Effectus Significantes in Reductionem Glucosi et Lipidorum
Tirzepatidum est primus agonista receptorii GIP/GLP-1 in mundo probatus, semel in hebdomada sumptus. Ut agonista receptorii dualis, simul et receptorem polypeptidi insulinotropici glucoso-dependentis (GIP) et receptorem GLP-1 in corpore humano ligare et activare potest. Praeter gradus glucosii imminuendos, cibi consumptionem, pondus corporis, et contentum adipum minuit, et usum lipidorum regulat. Praeter effectus suos significantes glucosii imminuendi et ponderis reducendi, analyses subgregum ex serie studiorum SURPASS demonstraverunt Tirzepatidum etiam indices metabolicos, ut pressionem sanguinis, lipida sanguinis, indicem massae corporis (BMI), et circumferentiam lumborum, emendare.
Approbationes Multinationales et Spes Promittentes
Secundum informationes pertinentes, medicamentum Mounjaro, medicamentum ad glucosum deprimendum, primum ab Administratione Civium et Medicamentorum (FDA) Civitatum Foederatarum mense Maio anni 2022 ad curationem adultorum diabete typi 2 laborantium probatum est. Deinde approbationes in Unione Europaea, Iaponia, aliisque regionibus accepit. Mense Novembri anni 2023, FDA etiam indicationem ad pondus amittendum sub nomine Zepbound probavit. Mense Maio anni 2024, feliciter mercatum Sinensem ingressus est. Ob latas applicationis possibilitates et validas investigationum probationes, Tirzepatidum unum ex praecipuis medicamentis peptidicis hodie factum est. Venditiones eius ad $5.163 miliarda anno 2023 pervenerunt, et primum quadrantem anni 2024 solum venditiones $2.324 miliarda vidit, incrementum mirabile ostendens.
De JYMed

Societas technologica Shenzhen JYMed (deinceps JYMed appellata) anno 2009 condita est, in investigatione, evolutione, productione, et venditione peptidorum et productorum peptido conexorum specializata. Cum uno centro investigationis et tribus maioribus basibus productionis, JYMed est unus ex maximis productoribus API peptidorum chemice synthesizatorum in Sinis. Turma principalis societatis in investigatione et evolutione plus quam viginti annos experientiae in industria peptidorum gloriatur et bis inspectiones FDA feliciter superavit. Systema industrializationis peptidorum comprehensivum et efficax JYMed clientibus seriem plenam servitiorum offert, inter quas evolutio et productio peptidorum therapeuticorum, peptidorum veterinariorum, peptidorum antimicrobialium, et peptidorum cosmeticorum, necnon subsidium registrationis et regulationis.
Actiones Negotiales Principales
1. Registratio domestica et internationalis API peptidicorum
2. Peptida veterinaria et cosmetica
3. Peptida ad usum accommodata et officia CRO, CMO, OEM
4. Medicamenta PDC (peptidum-radionuclidum, peptidum-molecula parva, peptidum-proteinum, peptidum-RNA)
Praeter Tirzepatidum, JYMed inscriptiones apud FDA et CDE pro pluribus aliis productis API, inter quas medicamenta classis GLP-1RA nunc popularia, ut Semaglutidum et Liraglutidum, submisit. Futuri clientes qui producta JYMed utuntur, numerum inscriptionis CDE vel numerum fasciculi DMF directe consulere poterunt cum inscriptiones ad FDA vel CDE submittunt. Hoc tempus ad documenta inscriptionis paranda requisitum, necnon tempus aestimationis et sumptum recensionis producti, significanter minuet.
Contacta Nos


Shenzhen JYMed Technologiae Societas, Ltd.
Inscriptio:Tabulata Octava et Nona, Aedificium I, Shenzhen Biomedical Innovation IndustrialPark, No. XIV Jinhui Road, Kengzi Subdistrictum, Pingshan District, Shenzhen
Telephonum:+86 755-26612112
Situs interretialis: http://www.jymedtech.com/
Tempus publicationis: XII Augusti, MMXXIV







