
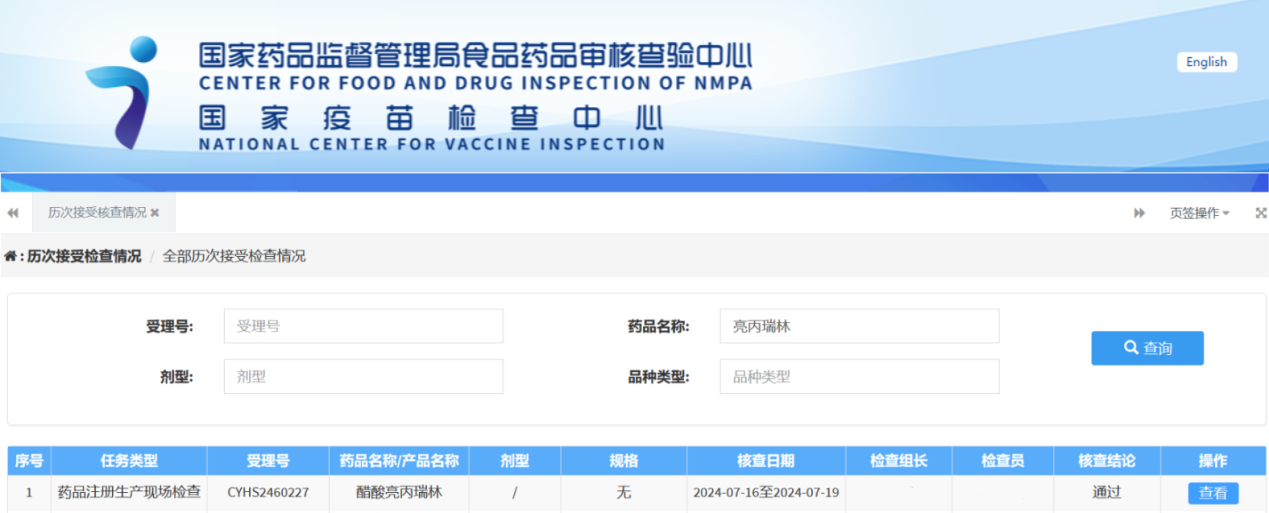
I kēia mau lā, ua hoʻolaha ʻo JYMed Technology Co., Ltd. i ka Leuprorelin Acetate, i hana ʻia e kāna ʻoihana ʻo Hubei JX Bio-Pharmaceutical Co., Ltd., ua holo kūleʻa i ka nānā ʻana i ka hoʻopaʻa inoa lāʻau.
Makeke Laau Laau Kumu
ʻO ka Leuprorelin Acetate kahi lāʻau injectable i hoʻohana ʻia e mālama i nā maʻi pili i ka hormone, me ke ʻano molekala C59H84N16O12•xC2H4O2. He gonadotropin-releasing hormone agonist (GnRHa) e hana ana ma ke kāohi ʻana i ka ʻōnaehana pituitary-gonadal. Hoʻokumu mua ʻia e AbbVie a me Takeda Pharmaceutical, kūʻai ʻia kēia lāʻau lapaʻau ma lalo o nā inoa inoa like ʻole ma nā ʻāina like ʻole. Ma ʻAmelika Hui Pū ʻIa, kūʻai ʻia ma lalo o ka inoa inoa LUPRON DEPOT, ʻoiai ma Kina, kūʻai ʻia ʻo ia ʻo Yina Tong.
Kaʻina Mākaʻikaʻi a me nā kuleana i wehewehe maikaʻi ʻia
Mai 2019 a 2022, ua pau ka noiʻi a me ka hoʻomohala ʻana i ka lāʻau lapaʻau, a ukali ʻia e ka hoʻopaʻa inoa ʻana o ka API ma Malaki 2024, i ka wā i loaʻa ai ka leka hoʻomaopopo. Ua hoʻoholo ʻia ka nānā ʻana i ka hoʻopaʻa inoa ʻana i ka lāʻau ma ʻAukake 2024. ʻO JYMed Technology Co., Ltd. ke kuleana no ka hoʻomohala ʻana i ke kaʻina hana, ka hoʻomohala ʻana i ke ʻano analytical, nā noiʻi haumia, ka hōʻoia ʻana i ka hale, a me ka hōʻoia ʻana i ke ʻano. ʻO Hubei JX Bio-Pharmaceutical Co., Ltd. ka luna o ke kaʻina hana hōʻoia, ka hōʻoia ʻana i ke ʻano loiloi, a me nā haʻawina kūpaʻa no ka API.
Hoʻonui i ka mākeke a me ka ulu ʻana o ka makemake
ʻO ka piʻi nui ʻana o ka maʻi prostate a me nā fibroids uterine ke alakaʻi nei i ka nui o ka noi no ka Leuprorelin Acetate. ʻO ka mākeke ʻAmelika ʻĀkau i kēia manawa ka luna o ka mākeke Leuprorelin Acetate, me ka ulu ʻana o ka mālama olakino a me ka ʻae kiʻekiʻe o nā ʻenehana hou ʻo ia ka mea hoʻokele ulu mua. I ka manawa like, ke hōʻike nei ka mākeke ʻĀsia, ʻo Kina, ke koi ikaika no ka Leuprorelin Acetate. Ma muli o kona pono, ke piʻi nei ka noi honua no kēia lāʻau lapaʻau, me ka nui o ka mākeke i manaʻo ʻia e hōʻea i ka $ 3,946.1 miliona e 2031, e hōʻike ana i ka nui o ka ulu ʻana o ka makahiki (CAGR) o 4.86% mai 2021 a 2031.
E pili ana iā JYMed

Ua hoʻokumu ʻia ʻo Shenzhen JYMed Technology Co., Ltd. (ma hope aku i kapa ʻia ʻo JYMed) i ka makahiki 2009, ʻoihana loea i ka noiʻi, hoʻomohala, hana, a kūʻai aku i nā peptides a me nā huahana pili i ka peptide. Me hoʻokahi kikowaena noiʻi a me ʻekolu kumu hana nui, ʻo JYMed kekahi o nā mea hana nui loa o nā peptide chemically synthesized API ma Kina. Ua hoʻokiʻekiʻe ka hui R&D koʻikoʻi o ka hui ma mua o 20 mau makahiki o ka ʻike ma ka ʻoihana peptide a ua lanakila ʻelua i ka nānā ʻana o FDA. Hāʻawi ka ʻōnaehana peptide industrialization a JYMed i nā mea kūʻai aku i nā lawelawe holoʻokoʻa, me ka hoʻomohala ʻana a me ka hana ʻana o nā peptides therapeutic, nā peptides holoholona, nā peptides antimicrobial, a me nā peptides cosmetic, a me ke kākau inoa ʻana a me ke kākoʻo hoʻoponopono.
Nā Hana Hana Nui
1. Ka hoʻopaʻa inoa ʻana i ka home a me ka honua o nā API peptide
2. Veterinary a me nā peptides cosmetic
3.Custom peptides a me nā lawelawe CRO, CMO, OEM
4.PDC nā lāʻau (peptide-radionuclide, peptide-liʻiliʻi mole, peptide-protein, peptide-RNA)
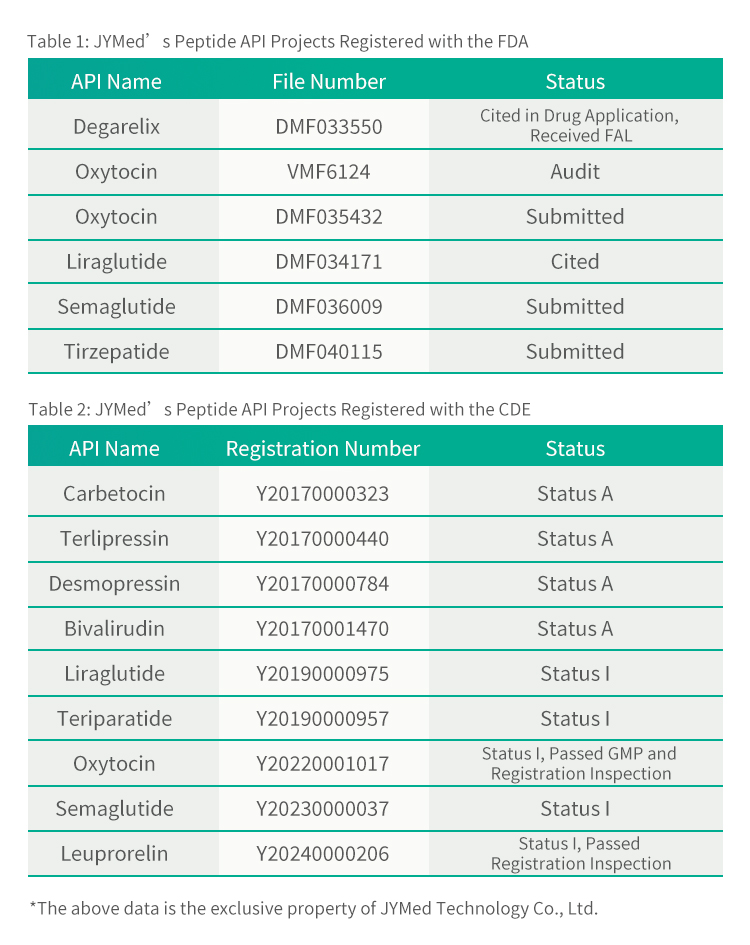
Ma kahi o Leuprorelin Acetate, ua hoʻouna ʻo JYMed i nā palapala hoʻopaʻa inoa me ka FDA a me CDE no nā huahana API ʻē aʻe, me nā lāʻau lapaʻau GLP-1RA kaulana i kēia manawa e like me Semaglutide、 Liraglutide a me Tirzepatide. Hiki i nā mea kūʻai aku e hoʻohana ana i nā huahana a JYMed ke kuhikuhi pololei i ka helu hoʻopaʻa inoa CDE a i ʻole ka helu faila DMF i ka wā e hoʻouna ana i nā noi hoʻopaʻa inoa i ka FDA a i ʻole CDE. E hoʻemi nui kēia i ka manawa e pono ai no ka hoʻomākaukau ʻana i nā palapala noi, a me ka manawa loiloi a me ke kumukūʻai o ka loiloi huahana.
Kāhea iā mā˚ou


Shenzhen JYMed Technology Co., Ltd.
Helu helu:8th & 9th Floors, Building 1, Shenzhen Biomedical Innovation Industrial Park, No. 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Kelepona:+86 755-26612112
Pūnaewele:http://www.jymedtech.com/
Ka manawa hoʻouna: ʻAukake-29-2024







