

Ua hauʻoli ʻo JYMed Technology Co., Ltd. i ka hoʻolaha ʻana ua hoʻopau pono kāna huahana, ʻo Tirzepatide, i ka hoʻopaʻa inoa ʻana i ka Drug Master File (DMF) me ka US FDA (DMF Number: 040115) a loaʻa iā ia ka FDA's Acknowledgment ma ʻAukake 2, 2024.
Hana Nui me ka maikaʻi kūpaʻa
Wahi a ka hoʻokele kiʻekiʻe o JYMed Technology, ʻo ka hana nui o Tirzepatide Active Pharmaceutical Ingredient (API) hiki ke piʻi i nā pae kilo. Paʻa a hoʻomau ʻia nā pūʻulu hana, me ka liʻiliʻi liʻiliʻi ma waena o nā pūʻulu, e hōʻoiaʻiʻo ana i ka maikaʻi.
Nā hopena koʻikoʻi i ka hoʻemi ʻana o ka glucose a me ka lipid
ʻO Tirzepatide ka mea mua loa o ka honua i ʻae ʻia i ka GIP/GLP-1 receptor agonist i hoʻokahi pule. Ma ke ʻano he agonist receptor ʻelua, hiki iā ia ke hoʻopaʻa a hoʻōla i ka glucose-dependent insulinotropic polypeptide (GIP) receptor a me ka GLP-1 receptor i loko o ke kino kanaka. Ma waho aʻe o ka hoʻohaʻahaʻa ʻana i nā pae glucose, hoʻemi ia i ka ʻai ʻana i ka meaʻai, ke kaumaha o ke kino, a me ka momona momona, a hoʻoponopono i ka hoʻohana lipid. Ma waho aʻe o kāna mau hopena hoʻohaʻahaʻa glucose nui a hoʻemi i ke kaumaha, ua hōʻike nā loiloi subgroup mai ka SURPASS series of study e hoʻomaikaʻi pū ana ʻo Tirzepatide i nā hōʻailona metabolic e like me ke koko, lipids koko, BMI, a me ka ʻāʻī o ka pūhaka.
Nā ʻae ʻana o ka lehulehu a me nā manaʻo hoʻohiki
Wahi a ka ʻike pili, ua ʻae mua ʻia ka Mounjaro hoʻohaʻahaʻa glucose e ka US FDA i Mei 2022 no ka mālama ʻana i nā pākeke me ka maʻi diabetes type 2. Ua loaʻa iā ia nā ʻae ʻia ma EU, Iapana, a me nā wahi ʻē aʻe. I Nowemapa 2023, ua ʻae pū ka FDA i ka hōʻailona pohō kaumaha ma lalo o ka inoa inoa Zepbound. I Mei 2024, ua komo maikaʻi ʻo ia i ka mākeke Kina. Hāʻawi ʻia i kāna mau noi noi ākea a me ka ʻikepili noiʻi kākoʻo ikaika, ua lilo ʻo Tirzepatide i kekahi o nā lāʻau peptide kaulana loa i kēia lā. Ua hōʻea kāna kūʻai ʻana i $ 5.163 biliona ma 2023, a ʻo ka hapaha mua o 2024 wale nō i ʻike i ke kūʻai ʻana o $ 2.324 biliona, e hōʻike ana i ka ulu nui o ka ulu ʻana.
E pili ana iā JYMed

Ua hoʻokumu ʻia ʻo Shenzhen JYMed Technology Co., Ltd. (ma hope aku i kapa ʻia ʻo JYMed) i ka makahiki 2009, ʻoihana loea i ka noiʻi, hoʻomohala, hana, a kūʻai aku i nā peptides a me nā huahana pili i ka peptide. Me hoʻokahi kikowaena noiʻi a me ʻekolu kumu hana nui, ʻo JYMed kekahi o nā mea hana nui loa o nā peptide chemically synthesized API ma Kina. Ua hoʻokiʻekiʻe ka hui R&D koʻikoʻi o ka hui ma mua o 20 mau makahiki o ka ʻike ma ka ʻoihana peptide a ua lanakila ʻelua i ka nānā ʻana o FDA. Hāʻawi ka ʻōnaehana peptide industrialization a JYMed i nā mea kūʻai aku i nā lawelawe holoʻokoʻa, me ka hoʻomohala ʻana a me ka hana ʻana o nā peptides therapeutic, nā peptides holoholona, nā peptides antimicrobial, a me nā peptides cosmetic, a me ke kākau inoa ʻana a me ke kākoʻo hoʻoponopono.
Nā Hana Hana Nui
1. Ka hoʻopaʻa inoa ʻana i ka home a me ka honua o nā API peptide
2.Vterinary a me nā peptides cosmetic
3.Custom peptides a me nā lawelawe CRO, CMO, OEM
4.PDC nā lāʻau (peptide-radionuclide, peptide-liʻiliʻi mole, peptide-protein, peptide-RNA)
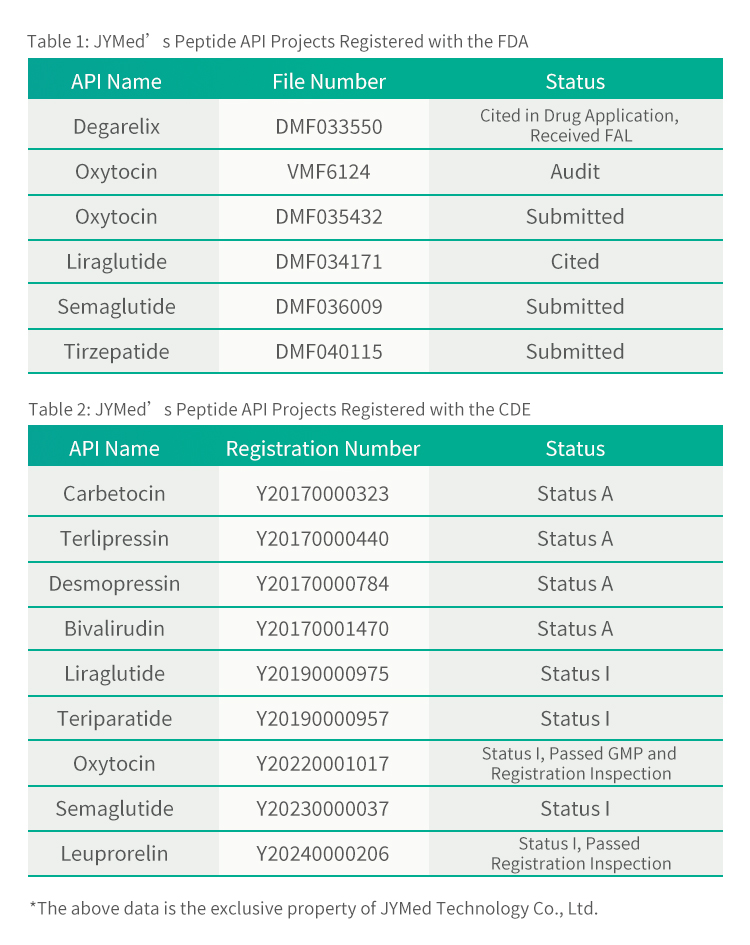
Ma kahi o Tirzepatide, ua hoʻouna ʻo JYMed i nā palapala hoʻopaʻa inoa me ka FDA a me CDE no nā huahana API ʻē aʻe, me nā lāʻau lapaʻau GLP-1RA kaulana i kēia manawa e like me Semaglutide a me Liraglutide. Hiki i nā mea kūʻai aku e hoʻohana ana i nā huahana a JYMed ke kuhikuhi pololei i ka helu hoʻopaʻa inoa CDE a i ʻole ka helu faila DMF i ka wā e hoʻouna ana i nā noi hoʻopaʻa inoa i ka FDA a i ʻole CDE. E hōʻemi nui kēia i ka manawa e pono ai no ka hoʻomākaukau ʻana i nā palapala noi, a me ka manawa loiloi a me ke kumukūʻai o ka loiloi huahana.
Kāhea iā mā˚ou


Shenzhen JYMed Technology Co., Ltd.
Helu helu:8th & 9th Papa, Hale 1, Shenzhen Biomedical Innovation IndustrialPark, No. 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Kelepona:+86 755-26612112
Pūnaewele: http://www.jymedtech.com/
Ka manawa hoʻouna: ʻAukake-12-2024







