

JYMed Technology Co., Ltd. ya yi farin cikin sanar da cewa samfurinsa, Tirzepatide, ya yi nasarar kammala rajistar Drug Master File (DMF) tare da US FDA (Lambar DMF: 040115) kuma ya sami Amincewar FDA a ranar 2 ga Agusta, 2024.
Samar da taro tare da Ingancin Barga
A cewar babban jami'in gudanarwa na Fasahar JYMed, yawan samar da sinadarin Tirzepatide Active Pharmaceutical Ingredient (API) na iya kaiwa matakan kilogiram. Batches na samarwa suna da ƙarfi kuma suna ci gaba, tare da ƙarancin bambance-bambance tsakanin batches, tabbatar da daidaiton inganci.
Muhimman Tasirin Glucose da Rage Lipid
Tirzepatide shine farkon yarda a duniya sau ɗaya-mako-mako GIP/GLP-1 agonist mai karɓa. A matsayin agonist mai karɓa na dual, yana iya ɗaure lokaci guda kuma kunna duka mai karɓar insulinotropic polypeptide (GIP) mai dogaro da glucose da mai karɓar GLP-1 a cikin jikin ɗan adam. Baya ga rage matakan glucose, yana rage cin abinci, nauyin jiki, da abun ciki mai mai, kuma yana daidaita amfani da lipid. Bayan gagarumin tasirin ragewar glucose da rage nauyi, ƙididdigar ƙungiyoyi daga jerin binciken SURPASS sun nuna cewa Tirzepatide kuma yana inganta alamun rayuwa kamar hawan jini, lipids na jini, BMI, da kewayen kugu.
Amincewa da Ƙasashen Duniya da yawa da Abubuwan Alƙawari
Dangane da bayanan da suka dace, Mounjaro mai rage glucose ya fara amincewa da FDA a cikin Mayu 2022 don kula da manya masu fama da ciwon sukari na 2. Daga baya ta sami izini a cikin EU, Japan, da sauran yankuna. A cikin Nuwamba 2023, FDA kuma ta amince da alamar asarar nauyi a ƙarƙashin sunan alamar Zepbound. A watan Mayun 2024, ya samu nasarar shiga kasuwar kasar Sin. Ganin fa'idodin aikace-aikacen sa da kuma bayanan bincike mai ƙarfi, Tirzepatide ya zama ɗaya daga cikin fitattun magungunan peptide a yau. Siyar da shi ya kai dala biliyan 5.163 a shekarar 2023, kuma kashi na farko na shekarar 2024 kadai ya ga tallace-tallacen dala biliyan 2.324, wanda ke nuna karuwar girma mai ban mamaki.
Game da JYMed

Shenzhen JYMed Technology Co., Ltd. (nan gaba ake magana a kai a matsayin JYMed) an kafa a 2009, ƙware a cikin bincike, ci gaba, samarwa, da kuma tallace-tallace na peptides da peptide alaka kayayyakin. Tare da cibiyar bincike guda ɗaya da manyan sansanonin samarwa guda uku, JYMed yana ɗaya daga cikin manyan masu samar da peptide API ɗin da aka haɗa ta sinadarai a cikin Sin. Babban ƙungiyar R&D na kamfanin yana alfahari sama da shekaru 20 na gogewa a cikin masana'antar peptide kuma ta sami nasarar wuce binciken FDA sau biyu. JYMed ta m da ingantaccen tsarin masana'antu peptide masana'antu yayi abokan ciniki cikakken kewayon ayyuka, ciki har da ci gaba da kuma samar da warkewa peptides, dabbobi peptides, antimicrobial peptides, da kwaskwarima peptides, kazalika da rajista da kuma kayyade goyon baya.
Babban Ayyukan Kasuwanci
1.Domestic da na duniya rajista na peptide APIs
2.Veterinary da na kwaskwarima peptides
3.Custom peptides da CRO, CMO, OEM ayyuka
4.PDC kwayoyi (peptide-radionuclide, peptide-kananan kwayoyin halitta, peptide-protein, peptide-RNA)
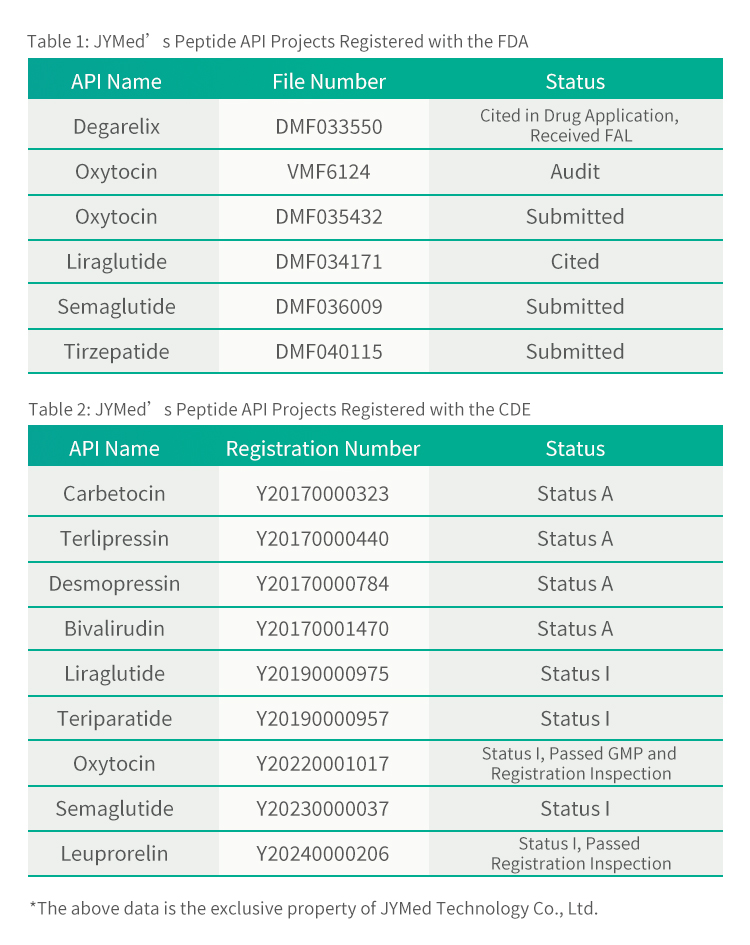
Baya ga Tirzepatide, JYMed ya ƙaddamar da takaddun rajista tare da FDA da CDE don wasu samfuran API da yawa, gami da shahararrun magungunan ajin GLP-1RA a halin yanzu kamar Semaglutide da Liraglutide. Abokan ciniki na gaba masu amfani da samfuran JYMed za su iya yin la'akari kai tsaye lambar rajistar CDE ko lambar fayil ɗin DMF lokacin ƙaddamar da aikace-aikacen rajista ga FDA ko CDE. Wannan zai rage mahimmancin lokacin da ake buƙata don shirya takaddun aikace-aikacen, da kuma lokacin kimantawa da farashin bitar samfur.
Tuntube Mu


Shenzhen JYMed Technology Co., Ltd.
Adireshi:benaye na 8 & 9, Ginin 1, Shenzhen Innovation Innovation Masana'antuPark, No. 14 Jinhui Road, Kengzi Subdistrict, Pingshan District, Shenzhen
Waya:+ 86 755-26612112
Yanar Gizo: http://www.jymedtech.com/
Lokacin aikawa: Agusta-12-2024







