Shenzhen JYMed Technology Co.,Ltd is a high-tech enterprise engaged in research and development, manufacturing and commercialization of peptides based products including active pharmaceutical ingredient peptides, cosmetic peptides, and custom peptides as well as new peptide drug development. JYMed has two wholly-owned subsidiaries: Shenzhen JXBio Pharmaceutical Co., Ltd. and Hubei JXBio Pharmaceutical Co, Ltd.
【R&D Center】
JYMed's R&D center, located in Shenzhen, is set up for the research and development of new drug substances, peptide APIs and relevant products. The center is equipped with modern peptide synthesizer, large-capacity preparative purification system, and comprehensive analytical instruments including MS, HPLC, GC, UV, IC etc. The R&D center also provides technology support for both new drug discovery and manufacturing process transferring.

【Production Bases】

Shenzhen JXBio site has two finished dose biological production lines that can provide commercial batches of small-capacity peptide injectables and freeze-dried powder products under cGMP guidelines. Hubei JXBio site is equipped with ten production lines for peptide API production and expanding more, making it one of the largest peptide API manufacturing bases in China.
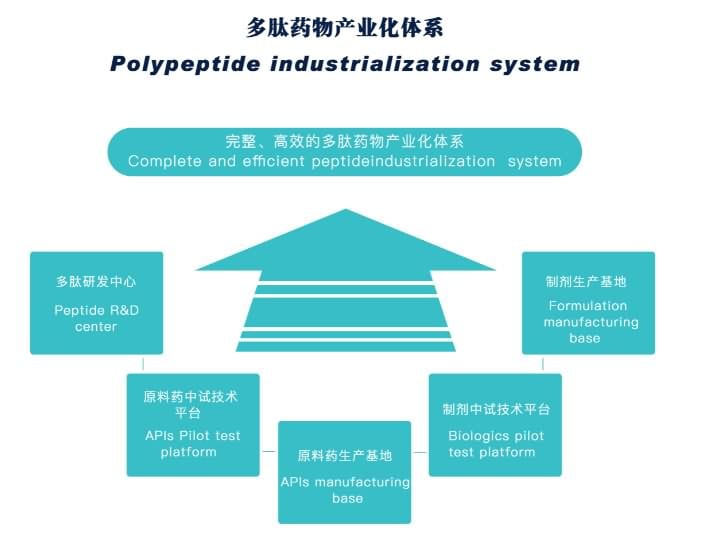
JYMed has a complete and efficient peptide industrialization system, and can provide comprehensive peptide services, including CRO/CMO/CDMO/OEM and regulatory affairs support, enabling us to be your reliable, independent, and proactive supplier for your peptides!


【 Factory pictures】









JYMed offers high quality peptide APIs, cosmetic peptides, and CRO/CMO services from research grade to cGMP grade with stringent quality control, helping you select the right protection for your application.






